Bavarian Nordic (BAVA DC/BVNRY)
Dendreon 2.0? Ineffective Cancer Vaccine Masked by Misleading Data
Disclosure
We are short shares of Bavarian Nordic A/S. Please click here to read full disclosures.
Sahm Adrangi, Chief Investment Officer, and Shane Wilson, Analyst, will host a conference call today at 11:00am EDT / 17:00 CEST to discuss the company’s report on Bavarian Nordic.
To participate in the conference call, please dial 855-780-5918 (domestic), 224-633-1732 (international), or 80-88-42-84 (Denmark) and reference the access code 2191536.
A replay of the call will be available following the call. To access the replay, contact acao@kerrisdalecap.com.
We are short shares of Bavarian Nordic A/S and believe that the stock is worth 70% less than the current trading value.
Our full report is available here.
Bavarian Nordic A/S (OMX: BAVA, OTC: BVNRY) is a $1.3B Danish vaccine-maker whose stock price has recently surged (up 63% YTD) thanks to excitement over its putative prostate-cancer treatment, Prostvac-VF, a therapeutic vaccine currently undergoing a Phase III clinical trial. Bavarian Nordic touts its earlier Phase II study of Prostvac as showing the “most pronounced survival to date in prostate cancer,” with an 8.5-month improvement in median overall survival, handily outperforming blockbuster drugs like Zytiga and Xtandi. The announcement in March that Bristol-Myers Squibb was paying $60mm upfront for an exclusive option to license and commercialize the vaccine gave investors great confidence that, despite the uncertainty surrounding any clinical trial, Prostvac is likely to succeed.
This confidence is misplaced. The often cited 8.5-month improvement is an illusion: treatment- arm survival was unexceptional relative to the results of other trials in similar patient populations, while placebo-arm survival was anomalously poor. This strikingly bad placebo performance likely had several causes, but one important one was age: relative to men who received Prostvac, those who received a placebo were much older – indeed, older than any group we have come across in any prostate-cancer clinical trial. Researchers have clearly and consistently found – as common sense would suggest – that elderly men with prostate cancer, compared to their younger counterparts, do in fact live substantially less long. Comparing an unexceptional treatment group to an anomalously bad placebo group is a good way to show a strong benefit where none truly exists.
More recent efforts to demonstrate improved survival in patients receiving both Prostvac and the cancer drug Yervoy only further underscore Prostvac’s inefficacy. In a 30-patient trial with no control group, across a range of Yervoy dose levels, median survival was 31.6 months – compared to the ~30-month survival seen over and over again in the control groups of other late-stage prostate-cancer studies, a negligible “improvement.” Given that Yervoy itself clearly has some standalone anti-tumor activity and has been shown to extend survival by (a non–statistically significant) 1.2 months even in post-chemo prostate-cancer patients, Prostvac’s combination-therapy data look even less impressive. The natural conclusion is that any apparent benefit comes from Yervoy; Prostvac itself accomplishes nothing.
This finding should come as no surprise: the history of therapeutic cancer vaccines is two decades of unmitigated failure. We expect nothing different from Bavarian Nordic.
Investment Highlights
- Prostvac’s purported 8.5-month survival benefit is an artifact of a bad control. In Prostvac’s Phase II study, which began in late 2003, the Prostvac group lived for 25.1 months; the placebo group lived for 16.6 months. For the relevant subset of patients – men with metastatic, castration-resistant prostate cancer (mCRPC) who are minimally symptomatic or asymptomatic – 25.1 months is an unremarkable outcome. For instance, in the TAX-327 study, initiated back in March 2000, men with minimal symptoms had median survival of 25.6 months. More recent studies have yielded even better results: the control groups in trials for tasquinimod, abiraterone, enzalutamide, and orterone, with enrollment start dates ranging from 2007 to 2010, survived 30.4, 30.3, 30.2, and 29.5 months in trials, respectively.
Since there is nothing special about Prostvac’s treatment-group performance, the purported benefit comes entirely from the anomalously bad performance of the placebo group. The original paper conceded that, applying a popular predictive model to a set of baseline prognostic factors, the treatment group had a 2.1-month survival “head start,” and an accompanying editorial further noted (emphasis added):
[I]t is of concern that the control group had a median overall survival lower than that predicted by the Halabi et al. model (16.6 months actual compared with 20.4 months predicted). The reasons for this discrepancy are not at all clear, particularly given the eligibility criteria designed to select lower-risk patients.
We hypothesize that an important source for this discrepancy is age: the median age in the Prostvac group was 71.5 years, while in the placebo group it was 79. We have found no group in any other prostate-cancer trial with an age distribution skewed so far to the right. The original paper’s authors dismiss this massive imbalance (favoring the Prostvac group) with the claim that “age is not a significant prognostic factor in prostate cancer,” citing the predictive model published in 2003 by Susan Halabi et al., but this claim is wrong. Halabi’s model was based on data from men with a relatively narrow range of ages, over which small differences may not matter.
By contrast, a study focusing specifically on elderly mCRPC patients (aged 75 years and older) showed that those in relatively good condition experience median survival of 17.5 months – very similar to the outcome for the Prostvac placebo group and approximately 10 months worse than that for younger men with similar disease characteristics. Another publication showed that mCRPC patients aged 85 years or older have 5-year survival rates that are less than a third of those for younger men aged 65-74, while a 2006 study by Halabi et al. noted that 60-to-69-year-olds experienced survival similar to that of 70-to-79-year-olds but lived almost twice as long as 80-to-89-year-olds, who constituted roughly half of the Prostvac Phase II placebo group.
In short, age does affect overall survival even for men with late-stage prostate cancer, likely explaining, at least in part, the Prostvac placebo group’s unusually bad performance. Regardless of the cause, though, there is no reason to expect such a bad control to recur in the larger Phase III study, set to finish in late 2016 or beyond; thus, there is no reason to expect Prostvac to show any benefit. The treatment will fail.
- Early combination-therapy data confirm the absence of a meaningful survival benefit. Although Prostvac’s Phase II study is inarguably the centerpiece of Bavarian Nordic’s case for the treatment’s efficacy, the company has recently touted a second, more recent study as demonstrating even greater potential benefits. In this Phase I trial – which had no control group – patients received the same dose of Prostvac and a range of doses of Yervoy (ipilimumab), an immune checkpoint inhibitor produced by Bristol-Myers. Across all doses, median overall survival was 31.6 months. Bavarian Nordic likes to portray this as a marked improvement over the Phase II trial’s 25.1-month survival, but in reality all comparable mCRPC trials have produced similar results for many years, even in placebo groups, stemming from better overall medical care and the wide range of other life-extending drugs available to trial participants once their condition deteriorates. Bavarian Nordic also likes to benchmark this survival result to the aforementioned Halabi 2003 predictive model, but this model, drawing on 15-year-old data, is now badly out of line with current clinical outcomes; indeed, it has since been superseded by an updated version that gives more optimistic predictions.
One striking illustration of both the inaccuracy of the Halabi 2003 predictive model and the mediocrity of the Prostvac/Yervoy data comes from a 2013 paper by Omlin et al. examining overall survival for all men with mCRPC participating in any clinical trial from 2003 to 2011 at one particular healthcare institution: the UK’s Royal Marsden Hospital. Men with no prior history of chemotherapy (termed “chemotherapy-naïve”) experienced median overall survival of 30.6 months, almost identical to the Prostvac/Yervoy result; meanwhile, the Halabi model predicted median survival of only 21 months, 31% worse than the actual outcome.
Below we take a graph from the Omlin paper, plotting percentage survival against time from referral (measured in months) for chemo-naïve Royal Marsden mCRPC patients, and superimpose a graph from Bavarian Nordic, plotting the same metrics for Prostvac/Yervoy combination-therapy patients. The survival curves are stunningly similar, especially considering the small size of the combination trial. Thus, using the Royal Marsden data as a surrogate “control” shows that the Prostvac recipients exhibit no advantage whatsoever. Consistent with the Phase II trial when taking into account age and other prognostic factors, Prostvac, even in combination with Yervoy, appears to accomplish nothing. Moreover, as the dark blue curve toward the left shows, the Halabi 2003 model is simply not a relevant reference point anymore: it badly underestimates expected survival, especially beyond the first year. It’s ludicrous for Bavarian Nordic to congratulate itself for clearing such a low bar.
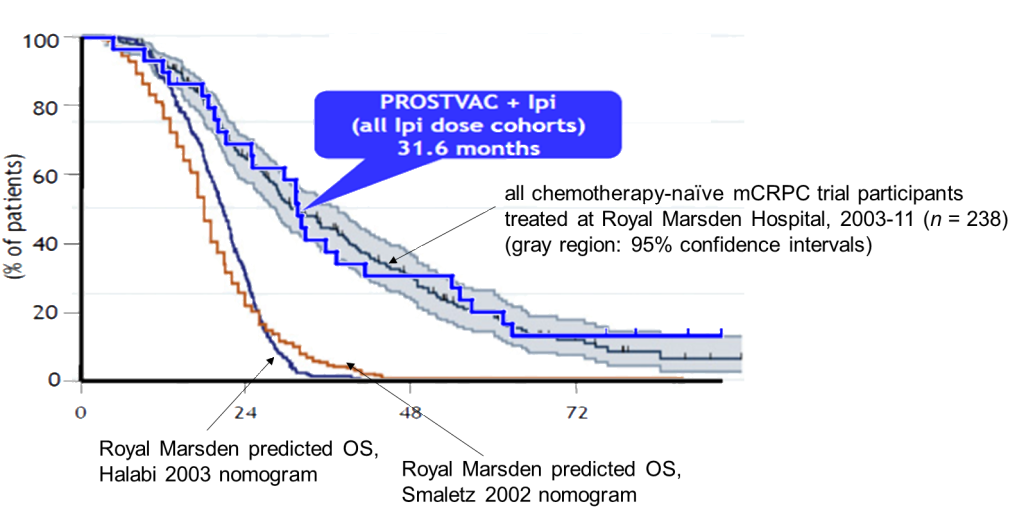
Source: Bavarian Nordic April 2015 investor presentation, Omlin et al. 2013, Kerrisdale analysis
Another relevant point of comparison is tasquinimod, a drug previously under development for mCRPC by Active Biotech and Ipsen. In a 201-patient Phase II trial, median overall survival was 33.4 months in the treatment group and 30.4 months in the placebo group, a statistically significant difference. In April, however, tasquinimod development was suspended when a 1,200-patient Phase III trial failed to show any survival benefit over placebo. If tasquinimod, despite its track record of 33.4 months of expected survival, can’t reliably outperform placebo, then Prostvac, with markedly less impressive results under its belt heading into Phase III, is bound to fail.
- The Prostvac goalposts keep moving. Back in 1999 when researchers conducted the first human trial of Prostvac, they highlighted that one of the six subjects saw his serum prostate-specific antigen (PSA), a widely used marker of prostate-cancer progression, plateau at a low level for months. In a 2000 follow-up, the highlight again was stable PSA levels and an apparent absence of disease progression in some patients. Thus the 125-patient Phase II trial had as its primary end point “progression-free survival,” i.e. time elapsed without the cancer becoming significantly worse. But these observations of supposedly improved disease progression – made without reference to any control group – disappeared with larger sample size, and Prostvac failed to deliver the benefit it was supposed to. Overall, Prostvac has shown no ability to shrink tumors or prevent PSA increases. The strange theory that Prostvac improves overall survival but not progression-free survival (or any other tangible measure of disease) was only cobbled together after the study was over.
Though some advocates suggest that an absence of progression benefit coupled with a real overall-survival benefit is typical of immunotherapy in general, this is false. The immune-checkpoint inhibitor ipilimumab, for instance – which failed to demonstrate a significant overall-survival increase in a large Phase III prostate-cancer study – did show a statistically significant 30% reduction in progression risk, and large PSA declines were 2.5x more frequent for ipilimumab patients than for placebo patients. To be sure, Dendreon’s prostate-cancer vaccine Provenge has a profile similar to Prostvac’s: a purported small survival benefit with no improvement in progression or other indicators of disease. But Provenge is not a happy precedent: despite its FDA approval in 2010, many clinicians harbored serious doubts about the evidence of its efficacy, and this pervasive skepticism (which continues to this day) was a key factor in Provenge’s commercial failure and Dendreon’s 2014 bankruptcy filing.
- Therapeutic cancer vaccines have a long history of failure. Harnessing the immune system to battle cancer is an exciting approach that, for certain patient populations, is finally starting to bear fruit. Sophisticated immunotherapies like checkpoint inhibitors and adoptive cell transfer have enjoyed some dramatic clinical successes. But simplistic therapeutic vaccines like Prostvac are another matter entirely; time and again, they have been tremendous disappointments. One review, published in 2004, compiled results from many different studies involving different cancers and different types of vaccines and concluded that “the overwhelming majority (>96%) of patients in the studies evaluated who received vaccine therapy for their underlying cancer did not exhibit objective evidence of cancer regression.” An updated review, published in 2011 under the title, “Therapeutic Cancer Vaccines: Are We There Yet?” looked at studies released in the intervening years and found the same 96% failure rate. A review by different authors, published in 2014 and focusing specifically on prostate-cancer vaccines, compiled 41 studies performed from 2000 to 2012 using a wide range of vaccine approaches, from viral vectors to plasmids to peptides, and including 1,100 patients in the aggregate – of whom only four enjoyed any tangible improvement in tumor burden. The authors summarize: “Vaccinations yielded immunological responses, but no study showed evidence for clinically relevant therapeutic improvement.” (In the case of Prostvac, the data are even less impressive, since even the immunological responses have been modest and inconsistent.)
Companies large and small have attempted to defy this track record of failure, only to fall flat on their faces:
- In 2008, Cell Genesys had to terminate two Phase III trials for its GVAX prostate-cancer vaccine when it became clear that the treatment conferred no survival benefit; after its stock price collapsed, the company was forced to sell itself.
- In 2012, Oxford BioMedica abandoned its TroVax prostate-cancer vaccine as a result of several factors: difficulties enrolling trial participants; a vast increase in the number of life-extending treatment options (like enzalutamide, abiraterone, and radium-223) available to men with late-stage prostate cancer; and early results for TroVax that gave no sign of meaningful efficacy.
- In 2013, GlaxoSmithKline announced that its Phase III trial of a melanoma vaccine had failed to extend disease-free survival in its targeted patient population.
- In 2014:
- Merck discontinued development of its non–small-cell lung-cancer vaccine tecemotide after it repeatedly failed to show any beneficial effect on overall survival or disease progression.
- GlaxoSmithKline abandoned the Phase III trial of its non–small-cell lung-cancer vaccine after it failed to show any benefit.
While Bristol-Myers’ willingness to pay $60mm upfront for an exclusive option on Prostvac was undoubtedly a vote of confidence – albeit on a very small scale, and with overall deal economics that imply a low probability of success – it would certainly not be the first time a large pharmaceutical company stumbled in this field. Moreover, the Bristol-Myers deal serves to cap any potential upside for Bavarian Nordic, since, in exchange for a series of additional payments that could total $915mm (but are likely to be substantially lower even if Prostvac succeeds), it has already traded away the vast majority of any future Prostvac revenue.
There is nothing special or innovative about Prostvac that would enable it to break the consistent pattern of failed cancer vaccines; to the contrary, the Prostvac concept is 20 years old. The entire approach of simply administering tumor-associated antigens and hoping for an effective immune response is a dead end.
- The scientific literature furnishes a multitude of convincing explanations for the failure of cancer vaccines. Why do therapeutic cancer vaccines fail to help patients even though they sometimes trigger a measurable immune response? One key factor is tolerance: the immune system has several mechanisms designed to prevent autoimmune attacks on self antigens like PSA, and the vast majority of nascent T cells targeting such antigens are killed before they ever exit the thymus. Another important factor is what scientists call the immunosuppressive tumor microenvironment. Tumors (and the immune system itself via negative-feedback mechanisms) blunt incipient T-cell attacks in myriad ways. One paper provided the following head-spinning overview:
Multiple layers of immune suppression are operational in the tumor environment, including other co-inhibitory molecules expressed on T cells such as PD-1/PD-L1, Tim-3, and LAG-3, Tregs, myeloid-derived suppressor cells, and soluble immunosuppressive mediators such as IDO (indoleamine 2,3-dioxygenase), arginase, prostaglandin E2 (PGE2), IL-6, IL-10, VEGF, and other cytokines and chemokines.
This list does not even include the possibility of “immune escape” as tumors evolve to downregulate the antigens that T cells have targeted. For example, even if Prostvac were to spark an initially effective immune response keyed on cells expressing PSA, the cancer might simply stop expressing PSA over time. This effect has been directly observed in a murine model of prostate cancer: researchers injected antigen-specific T cells into the mice and observed them killing off antigen-positive tumor cells, but this only led to the outgrowth of antigen-negative tumor cells and had no impact on the overall progression of the tumor.
Even the formidable challenges of immunosuppression and immune escape – which have foiled treatments far more robust than Prostvac – presuppose that T cells actually manage to traffic into the tumor, another major difficulty. They also presuppose that the immune system targets the desired recombinant antigen rather than simply focusing on the viral vector itself. Yet research suggests that T-cell responses to recombinant viral vectors – i.e. the viruses themselves, not their payload of foreign antigens (PSA in the case of Prostvac) – can be 20 to 30 times more intense than responses to the foreign antigens. This finding, an instance of the broader phenomenon of “immunodominance,” is consistent with Prostvac data showing derisory PSA-specific T-cell responses in vaccinated patients; by contrast, typical responses to the vaccinia virus itself, one of the vectors for Prostvac, are an order of magnitude stronger. The immune system is fighting the vaccine far more diligently than it is attempting to fight the cancer.
Finally, since prostate cancer is primarily a disease of older men, Prostvac also faces the problem of “immunosenescence”: immune responses across the board tend to deteriorate with age. Indeed, even conventional prophylactic vaccines like the flu vaccine are much less effective in elderly populations. Therapeutic cancer vaccines attempting to overcome strong barriers to autoimmunity and target “self” antigens like PSA are unlikely to fare any better.
In short, a wide array of mechanisms limits the potential effectiveness of therapeutic cancer vaccines, including tolerance, immunosuppression, immunodominance, and, for prostate cancer, immunosenescence. Prostvac is a weak agent that fails to address these daunting challenges, so it should be no surprise that it can’t.
- Bavarian Nordic’s core business is at risk. While market enthusiasm for Bavarian Nordic centers on Prostvac, the company’s only material revenue source in the past several years has been the sale of Imvamune, a weaker form of the conventional smallpox vaccine intended for people with compromised immune systems, to the US government for its “strategic national stockpile.” Notwithstanding the vaccine’s high cost and unknown efficacy – the objects of criticism from some of the world’s leading smallpox experts, including the World Health Organization – the government appears committed to working with Bavarian Nordic to finalize a new, longer-lasting freeze-dried version. But given the likely dramatic increase in shelf life and thus dramatic decline in the need for future replenishment, revenue from the freeze-dried vaccine will effectively be non-recurring, putting this business’s sustainability in doubt. Moreover, recently published research not only demonstrated the similarity of the liquid and freeze-dried formulations but also showed that, with a different route of administration (intradermal rather than subcutaneous), only 20% of the conventional dose could achieve the same level of protection, implying that the Strategic National Stockpile could purchase 5x less material from Bavarian Nordic yet cover the same target population. At best, this discovery will damage the company’s bargaining position; at worst, it will decimate its future revenue.
Investors have come to view Bavarian Nordic as a de-risked bet on a very promising agent, with the purported 8.5-month survival improvement at the heart of the long thesis. One sell-side firm has gushed, “A significant Phase II survival benefit suggests PROSTVAC immunotherapy has the potential to revolutionise prostate cancer treatment.” But Prostvac will revolutionize nothing. After taking into account the profound flaws of the Phase II trial as well as the weakness of the early combination-therapy results, it’s clear that Prostvac is just as ineffective as every other failed cancer vaccine. Bavarian Nordic did not stumble onto the magical key to making this doomed approach work; it merely got lucky with a statistical fluke.
Please read our here for our full analysis.





Add New Comment